The first law is concerned with the internal energy change of a system. This is what ΔU represents. As I admitted earlier U is an odd concept to me. I think this primarily arises because, at least chemically speaking, we're mostly interested in the changes in U. Changes in energy I am comfortable with, and the way in which they change is what the right hand side of the equation describes: namely, q and W. These two symbols designate every process that could possibly change the internal energy of a given system.
"W" stands for work. Work can encompass a large number of things. Work, mechanically speaking, is defined as

In a mathematical context, and in one dimension. This simply states that a Force (From Newton's 2nd Law F = ma) applied over a distance equals the amount of work done. If you happen to be unfamiliar with integration, the long squiggly sign is the mathematical way of saying "from point A to point B", and "dx" means the change in position x (like a Cartesian grid, such as you learn about in algebra class). Work can come from more than mechanics, though: It can be performed by electric circuits, or chemical reactions (cell phones, vehicles), or some other type. In the end, however, it's still work.
Thermodynamics is largely used to describe gas phase systems. This doesn't have to be the case, but the gas phase is effected by thermodynamics more so than solid and liquid phases, at least with respect to the phases we normally encounter. As such, work is not defined in the mechanical sense. Instead the concepts of pressure, volume, and temperature are used, and work is defined as

Where P is Pressure and dV is the change in volume. The reason for the negative sign, in this context, is a matter of definition. When "negative" work is performed, this indicates that the system of interest (in this case, a gas) is loosing energy (or releasing energy, as an equivalent expression) to the surroundings. If the integration produces a positive sign (by having a negative PV), then this indicates that energy is entering the system. This is actually analogous to the above definition as mechanical work almost always gives energy to the system: A force applied to a ball from point a to point b will give energy to that ball. But in the context of gas description the application of a force would decrease the volume of a system, which mathematically would give a negative "PV", which goes against the convention of negative = release energy from system, and positive = give energy to system.
Looking at this, the "process" part doesn't seem to be coming into play at all. If you go from point A to point B, won't the distance between these points be the same regardless? As stated so far, it seems that way, but there's one other aspect of integration that works into the idea of "Process" here. An equivelent way to look at integration is that it gives you the area underneath a curve on a Cartesian grid. For example, this:

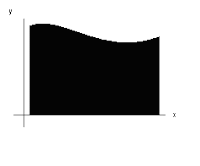
would integrate to give the area of the black shaded block here:

As such, integrating a function like this:
Would give a much larger area in comparison to the first one, as can be seen here:
Sorry for the math digression, but I think it's important to understanding the concept of processes. When I took Chem I, the whole "you can go different ways to the top of a mountain" shpeal only served to confuse me further.
The great thing is there is such a function that describes gases and relates to our function for work. It is known as the ideal gas law: PV = nRT. In most thermodynamic cases, n is constant (and stands for the number of particles), and in all cases, R is constant (Specifically, named "The Gas Constant). Rearranging this algebraically gives

Which states that P is a function of T and V, or P(T, V). We can plot this in three dimensions, but there's no need: If you have two of the numbers from above, whether it be pressure, temperature, or volume, then you can find the third as n and R are held constant. Also, since we're interested in work, we might as well label one axis and pressure and the other as volume since those are the two variables that determine work. I'm not sure why this is the case, but I've never seen it otherwise, so I'll state that "By convention" the x axis is volume, and the y axis is pressure, thereby giving
Now, analogously to the Cartesian plane of algebra, every point on here is defined by some number, but here the number has a unit, or a meaning, attached to it: Namely, the pressure or the volume associated. Also analogously to the Cartesian plane above, if you "integrate" from one point to another on this plane, you will obtain the area associated with it. If you recall, integration from "Point A to Point B" [Or, rather, from point (Vo, Po) to point (V, P)] was also the definition of work. In other words, the area of a block you would obtain by moving from one point on the plane to another is equal to the amount of work performed. The actual path that one takes is the process one uses to get from one point on this plane to another. Therefore, the process described by this path:

Takes (or releases, depending on which point you start with) less energy than this path:

That is why work depends upon the process taken: Because it is the area underneath the curve which would be drawn from one point on the Cartesian Plane of Pressure-Volume to another point. If you were to decrease pressure and then increase volume, you'd be doing less work. If you instead increased pressure before increasing volume, you'd be doing more work.
Each of these paths have special names attached to them that designate what's happening. The first PV-integration example is called "isothermal", meaning that temperature does not change in the process, and the second is "isobaric" meaning that pressure does not change in the process. Some other processes I can think of are "isochoric", which means that volume is held constant (No work done), and "adiabatic" which means that energy is not lost or gained through the other process involved in determining the change in internal energy: Heat (q). I'd go into heat, but I think this one is long enough as it is, so I'll reserve that for next time around.
 since products are so highly favored. However, if the equilibrium constant is smaller than one, then reactants are more favored than products in this particular chemical reaction.
since products are so highly favored. However, if the equilibrium constant is smaller than one, then reactants are more favored than products in this particular chemical reaction.
